Which Of The Following Helps Prevent Changes In The Acid-base Balance Of Body Fluids
In this commodity we volition hash out most:- 1. Definition of Acid-Base of operations Homeostasis 2. Response to an Acrid-Base of operations Imbalance 3. Regulation.
Definition of Acid-Base Homeostasis:
Acid-base of operations homeostasis is the part of human being homeostasis concerning the proper residuum between acids and bases, in other words, the pH. The body is very sensitive to its pH level, so stiff mechanisms exist to maintain it. Outside the acceptable range of pH, proteins are denatured and digested, enzymes lose their ability to function, and death may occur.
What is pH?
The term pH refers to the negative log of hydrogen ion concentration-
pH = log 1/H+ = – log [H+]
Normal H+ is twoscore nEq/Fifty.
Normal pH is
pH = – log [0.00000004]
pH = 7.iv
The normal range of blood pH falls between seven.35 and seven.45 and our Acid-base of operations balance has to maintain pH within this normal range.
pH of Some Body Fluids:
Arterial blood – 7.4
Venous blood – seven.35
Interstitial fluid – 7.35
ICF – 6 – 7.4
Urine – 4.five – eight
Gastric HCl – 0.viii
Survival range of pH is – 6.8 to 8.0
Acrid:
An acrid is a molecule containing hydrogen atom that can release hydrogen ions in solutions, east.g.
HCI → H+ + Cl–
H2CO3 → H+ + HCO3
At that place is always a constant product of acid past the trunk's metabolic processes and to maintain balance, these acids demand to be excreted or metabolized. The various acids produced past the body are classified as respiratory (or volatile) acids and metabolic (or stock-still) acids.
Respiratory Acid:
The acrid is more than correctly carbonic acrid (HiiCO3) simply the term 'respiratory acrid' is usually used to mean carbon dioxide. Carbon dioxide is the end-product of complete oxidation of carbohydrates and fatty acids. It is chosen a volatile acid meaning in this context it tin be excreted via the lungs. Of necessity, considering the amounts involved at that place must be an efficient system to rapidly excrete CO2.
Metabolic Acids:
This term covers all the acids the torso produces which are nonvolatile. Considering they are not excreted by the lungs they are said to be 'stock-still' in the body and hence the culling term fixed acids. All acids other than HtwoCO3 are stock-still acids.
For Acid-base balance, the amount of acid excreted per solar day must equal the amount produced. The routes of excretion are the lungs (for CO2) and the kidneys (for the stock-still acids).
Acid-Base Imbalance:
Acid-base of operations imbalance occurs when a significant insult causes the blood pH to shift out of the normal range (7.35 to seven.45). An backlog of acid is called acidosis (pH less than 7.35) and an excess of base of operations is called alkalosis (pH greater than 7.45). The process that causes the imbalance is classified based on the etiology of the disturbance (respiratory or metabolic) and the direction of change in pH (acidosis or alkalosis).
There are four basic processes:
(i) Metabolic acidosis,
(2) Respiratory acidosis,
(iii) Metabolic alkalosis, and
(iv) Respiratory alkalosis.
One or a combination may occur at any given time.
Response to an Acid-Base Imbalance:
The body'south response to a change in Acid-base condition has three components:
one. Offset Defence force:
Buffering.
2. 2d Defence:
Respiratory compensation by alteration in arterial PCOii
3. Tertiary Defence:
Renal compensation by alteration in HCO3 – excretion.
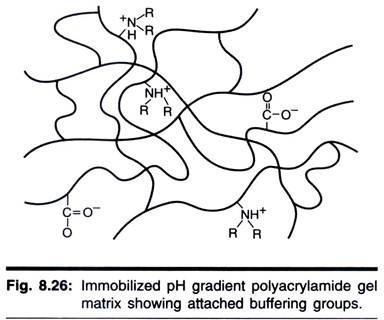
Buffer:
Body Fluid:
A buffer is any substance that can reversibly demark H+. Buffer + H+ ↔ H buffer
80 mEq of H+ are produced per day.
ane. Bicarbonate Buffer System:
The major buffer system in the ECF is the COii-bicarbonate buffer system. This is responsible for most 80% of extracellular buffering simply information technology cannot buffer respiratory Acid- base of operations disorders.
2. Phosphate Buffer Systems:
The phosphate buffer systems are not important blood buffer as its concentration is also depression. It plays an important role in renal tubular system.
3. Hemoglobin:
Protein buffers in blood include hemoglobin (150 g/ane) and plasma proteins (70 k/1). Buffering is past the imidazole group of the histidine residues. Hemoglobin is quantitatively about 6 times more important than the plasma proteins as it is present in about twice the concentration and contains virtually three times the number of histidine residues per molecule. For instance, if blood pH inverse from seven.5 to six.5, hemoglobin would buffer 27.5 mmol/1 of H+ and total plasma poly peptide buffering would account for but four.ii mmol/1 of H+. Deoxyhemoglobin is a more effective buffer than oxyhemoglobin.
'Whenever, there is a modify in H+ concentration in the ECF, the balance of all the buffer systems changes at the same time'.
H+ = K1 × HA1/A1 = K2 × HA2/A2 = K3 × HA3/A3.
K1, K2 and K3 are dissociation constants of 3 respective acids.
Regulation of Acrid-Base of operations Balance:
I. Respiratory Regulation of Acid-Base Residue:
i. Regulates H+ concentration through CO2 in ventilation.
2. ↑ [H+] → ↑ alveolar ventilation
3. Buffering ability of respiratory arrangement is 1-2 times greater than chemical buffers.
iv. Lung diseases decrease the efficacy of the buffering power. Respiratory regulation refers to changes in pH due to PCO2 changes by altering the ventilation. This change in ventilation tin occur apace with significant effects on pH. Carbon dioxide is lipid soluble and crosses jail cell membranes rapidly, so changes in PCO2 result in rapid changes in [H+] in all body fluid compartments.
II. Renal Regulation of Acid-Base Balance:
There are three systems that regulate H+ concentration in the body fluids to prevent acidosis or alkalosis.
ane. The chemic Acid-base buffer systems which combine with acid or base to prevent excessive changes in H+ concentration.
2. The respiratory centers which regulates the removal of COtwo from ECF.
3. The kidneys regulate blood pH past iii mechanisms.
i. The chemical Acid-base buffer systems which combine with acrid or base of operations to prevent excessive changes in H+ concentration.
two. The respiratory centers which regulates the removal of CO2 from ECF.
3. CO2 + H2O ↔ H2CO3
a. Excretion of acid in the form of titrable acrid and ammonium ions.
b. Reabsorption of the filtered HCOthree
c. Generation of new NaHCO3
Machinery of H+ Secretion by PT:
i. Formation of carbonic acid
2. Secretion of H+ into the lumen via Na+ H+ counter-transport in luminal membrane—an example of secondary agile transport.
iii. H+ secreted in the lumen combines with filtered HCO3 and helps in reabsorption.
iv. HCOthree formed in the cell diffuses into interstitial fluid through basolateral membrane. This is done by Na HCOthree transport and CI HCOiii exchanger. Thus for each H+ secreted 1 Na+ and one HCO3 ion enter the interstitial fluid.
Fate of H ion into the Lumen:
1. Nontitrable acidity
2. Titrable acidity
1. Nontitrable Acidity:
H+ ion combines with HCOthree and NH3 producing non-titrable acids. The reactions are:
The procedure by which NHthree is secreted into the urine and and then changed to NH4 maintaining the concentration gradient for improvidence of NHthree is called nonionic diffusion.
Ammonium Ion Secretion:
Glutamine is metabolized in PCT cells yielding ammonium and bicarbonate. The NH4 + is actively secreted past Na+ NHiv + pump and bicarbonate is returned to blood.
Ammonium Ion Secretion in CD:
The CD is permeable to NH3 which diffuses into tubular lumen but less permeable to NHfour, therefore, NH4 is trapped in the tubular lumen and excreted in the urine.
two. Titrable Acidity:
The H+ ions that combine with dibasic phosphate produce monobasic phosphate which contributes titrable acerbity.
Internet acid secretion = Titrable + urinary NH4 – urinary HCO3 acidity
Full acid excreted by the kidney = fifty to 100 mEq/day.
Mechanism of H+ Secretion by DT and CD is independent of Na+:
i. ATP driven pumps increment H+ concentration by thousand times. Aldosterone acts on this pump to increase H+ secretion.
ii. H+ K+ ATPase is also responsible.
Reabsorption of Filtered HCO3:
PT reabsorbs eighty% of filtered HCO3, H+ secreted in lumen of PT combines with HCO3 to form H2CO3. It is converted to COtwo and H2O. CO2 diffuses into tubular cells. CO2 combines with H2O to form H2CO3 which dissociates into H+ and HCOiii.
The H+ ion is secreted into tubule and HCO3 ion diffuses into interstitial fluid. When each molecule of HCOthree is reabsorbed into lumen, one molecule of HCOiii diffuses into claret fifty-fifty though it is non the same molecule. The pH of fluid in proximal tubule is very little altered since the H+ ion secretion is neutralized past HCO3 ion reabsorption.
LOH reabsorbs 15% of filtered HCO3.
DT and CT reabsorb only five% of the filtered HCO3.
Generation of New NaHCOthree Ions:
Phosphate and ammonia buffers in the tubule carries excess H+ ions to generate new NaHCO3 ions. Therefore, whenever H+ ion secreted into the tubules combines with a buffer other than HCO3, the cyberspace event is addition of new bicarbonate to the blood. For examples if H+ reacts with NHthree to form NH4, NH4 is trapped in the tubular lumen and eliminated in the urine. For each NHiv excreted, a new HCOthree is generated and added to the blood.
Kidneys filter 4320 mEq of HCOthree –/solar day.
(180 50 × 24 mEq/L)
To reabsorb 4320 mEq of HCOthree, equal amount of H+ are secreted.
In addition, 80 mEq of H+ from nonvolatile acids are likewise secreted, making the full of 4400 mEq of H+ to be secreted/day. Only a small amount of backlog H+ can exist secreted in ionic form in the urine. Minimal urine pH is virtually four.5 corresponding to an H+ concentration of 0.03 mEq/Fifty. For every liter of urine but 0.03 mEq of H+ tin can exist excreted.
To excrete lxxx mEq of H+, 2667 liters of urine would take to be formed.
Acidosis:
Defined as an increment in H+ concentration or decrease in pH (<7.iv).
Alkalosis:
Defined as a decrease in H+ concentration or increase in pH (>vii.4).
Metabolic:
Whatever disturbance of Acid-base balance resulting from changes in HCOiii – concentration in ECF.
Metabolic Acidosis:
Decrease in plasma HCOiii –
Metabolic Alkalosis:
Increase in plasma HCO3 –.
Respiratory:
Disturbances in Acid-base residuum due to changes in PCO2.
Respiratory Acidosis:
Increment in PCOtwo
Respiratory Alkalosis:
Decrease in PCO2.
Renal Correction:
Alkalosis:
Either a decrease in tubular secretion of H+ or increased excretion of HCO3 –.
Acidosis:
Either an increase in excretion of H+ or by generation of new HCO3 –.
Respiratory Acidosis:
Whatever factor that decreases the charge per unit of pulmonary ventilation increases the PCO2 of ECF → ↑ H2CO3 → ↑ H+.
Causes:
i. Harm to respiratory center.
two. Obstruction of passages of the respiratory tract.
iii. Pneumonia.
four. Emphysema.
Respiratory Alkalosis:
Caused by:
i. Hyperventilation.
ii. Physiologically at high altitudes.
three. Psychoneurosis.
Metabolic Acidosis:
Causes:
i. Failure of kidneys to excrete metabolic acids.
ii. Formation of excess quantities of metabolic acids.
iii. Addition of metabolic acids to the trunk.
iv. Loss of base from the body.
Renal Tubular Acidosis:
i. Chronic renal failure.
ii. Addison's illness.
iii. Fanconi'due south syndrome.
four. Diarrhea.
v. Vomiting of intestinal contents.
half-dozen. Diabetes mellitus.
vii. Ingestion of acids (aspirin, methyl alcohol).
Metabolic Alkalosis:
Causes:
i. Excess retention of HCO3 –.
two. Loss of H+ from the body.
three. Apply of diuretics (except carbonic anhydrase inhibitor).
iv. Backlog aldosterone.
v. Airsickness of gastric contents.
vi. Ingestion of element of group i drugs.
7. Treatment of acidosis.
8. Oral NaHCO3.
ix. Infusion of sodium lactate and sodium gluconate.
Treatment of Alkalosis:
i. Oral ammonium chloride.
2. Lysine monohydrochloride.
Renal Correction:
↓ Tubular section of H+ ions.
↑ Excretion of HCO3 ions.
Acrid-Base Nomogram:
To diagnose acid-base disorders quickly and to observe out the severity. pH, PCO2 and HCOiii values are used. Sufficient time should be given for compensatory response. 6-12 hours for lungs and 3-5 days for kidneys.
Nomogram:
Anion Gap:
It is the measure of difference betwixt unmeasured anions and cations.
= (Na+) – (HCOiii-) (Cl-)
= 144 – 24 – 108
= 12 mEq/Fifty
Unmeasured cations are Ca++, Mg++ and K+.
Unmeasured anions are albumin, PO4, Theniv, etc.
Normal range is 8-16 mEq/50.
Conditions Associated with Increased Anion Gap:
i. DM
ii. Lactic acidosis
iii. Chronic renal failure
iv. Starvation
v. Aspirin poisoning
Conditions associated with Decreased Anion Gap:
i. Diarrhea.
ii. Renal tubular acidosis.
iii. Carbonic anhydrase inhibitors.
iv. Addison's illness.
Source: https://www.biologydiscussion.com/human-physiology/human-excretory-system/acid-base-homeostasis-response-regulation-excretory-system-biology/85276
Posted by: lindleyadind1979.blogspot.com


0 Response to "Which Of The Following Helps Prevent Changes In The Acid-base Balance Of Body Fluids"
Post a Comment